science

Sputnik 1 (/ˈspʌtnɪk, ˈspʊtnɪk/, ‹See Tfd›Russian: Спутник-1, Satellite 1) was the first artificial Earth satellite. It was launched into an elliptical low Earth orbit by the Soviet Union on 4 October 1957 as part of the Soviet space program. It sent a radio signal back to Earth for three weeks before its three silver-zinc batteries became depleted. Aerodynamic drag caused it to fall back into the atmosphere on 4 January 1958. The world's first observation was made at the school observatory in Rodewisch (Saxony). It was a polished metal sphere 58 cm (23 in) in diameter with four external radio antennas to broadcast radio pulses. Its radio signal was easily detectable by amateur radio operators, and the 65° orbital inclination made its flight path cover virtually the entire inhabited Earth. The satellite's success was unanticipated by the United States. This precipitated the American Sputnik crisis and triggered the Space Race, part of the Cold War. The launch was the beginning of a new era of political, military, technological, and scientific developments. The word sputnik is Russian for satellite when interpreted in an astronomical context; its other meanings are spouse or traveling companion. Tracking and studying Sputnik 1 from Earth provided scientists with valuable information. The density of the upper atmosphere could be deduced from its drag on the orbit, and the propagation of its radio signals gave data about the ionosphere. Sputnik 1 was launched during the International Geophysical Year from Site No.1/5, at the 5th Tyuratam range, in Kazakh SSR (now known as the Baikonur Cosmodrome). The satellite traveled at a peak speed of about 8 km/s (18,000 mph), taking 96.20 minutes to complete each orbit. It transmitted on 20.005 and 40.002 MHz, which were monitored by radio operators throughout the world. The signals continued for 22 days until the transmitter batteries depleted on 26 October 1957. On 4 January 1958, after three months in orbit, Sputnik 1 burned up while reentering Earth's atmosphere, having completed 1,440 orbits of the Earth, and travelling a distance of approximately 70,000,000 km (43,000,000 mi). **Megathreads and spaces to hang out:** - 📀 Come listen to music and Watch movies with your fellow [Hexbears nerd, in Cy.tube](https://live.hexbear.net/c/movies) - 🔥 Read and talk about a current topics in the [News Megathread](https://hexbear.net/post/3311273) - ⚔ Come talk in the [New Weekly PoC thread](https://hexbear.net/post/3468550) - ✨ Talk with fellow Trans comrades in the [New Weekly Trans thread](https://hexbear.net/post/3363552) - 👊 Share your gains and goals with your comrades in the [New Weekly Improvement thread](https://hexbear.net/post/3569281) **reminders:** - 💚 You nerds can join specific comms to see posts about all sorts of topics - 💙 Hexbear’s algorithm prioritizes comments over upbears - 💜 Sorting by new you nerd - 🌈 If you ever want to make your own megathread, you can reserve a spot [here nerd](https://hexbear.net/post/261657) - 🐶 Join the unofficial Hexbear-adjacent Mastodon [instance toots.matapacos.dog](https://toots.matapacos.dog/explore) **Links To Resources (Aid and Theory):** **Aid:** - 🌈 [LGBTQ+ Resource Post](https://hexbear.net/post/279079) - 🍉 [Resources for Palestine](https://buildpalestine.com/2021/05/15/trusted-organizations-to-donate-to-palestine/) - [🐌☕ Zapatista Coffee](https://schoolsforchiapas.org/store/coffee-corn-and-agricultural/zapatista-coffee/) **Theory:** - ❤️[Foundations of Leninism](https://www.marxists.org/reference/archive/stalin/works/1924/foundations-leninism/index.htm) - ❤️[Anarchism and Other Essays](https://theanarchistlibrary.org/library/emma-goldman-anarchism-and-other-essays)
 arstechnica.com
arstechnica.com
>We tend to think of agriculture as a human innovation. But insects beat us to it by millions of years. Various ant species cooperate with fungi, creating a home for them, providing them with nutrients, and harvesting them as food. This reaches the peak of sophistication in the leafcutter ants, which cut foliage and return it to feed their fungi, which in turn form specialized growths that are harvested for food. But other ant species cooperate with fungi—in some cases strains of fungus that are also found growing in their environment. > >Genetic studies have shown that these symbiotic relationships are highly specific—a given ant species will often cooperate with just a single strain of fungus. A number of genes that appear to have evolved rapidly in response to strains of fungi take part in this cooperative relationship. But it has been less clear how the cooperation originally came about, partly because we don't have a good picture of what the undomesticated relatives of these fungi look like. > >Now, a large international team of researchers has done a study that traces the relationships among a large collection of both fungi and ants, providing a clearer picture of how this form of agriculture evolved. And the history this study reveals suggests that the cooperation between ants and their crops began after the mass extinction that killed the dinosaurs, when little beyond fungi could thrive.
 www.nature.com
www.nature.com


From the book [*The Machinery of Life*](https://ccsb.scripps.edu/goodsell/machinery-of-life-reducedillustrations/) by David S. Goodsell. You can read about the artist's process [here](https://journals.uic.edu/ojs/index.php/jbc/article/view/6627/5251). This is an illustration of the first stage of the [central dogma of molecular biology](https://en.wikipedia.org/wiki/Central_dogma_of_molecular_biology), often stated simplistically as "DNA makes RNA, and RNA makes protein". Our DNA stores the blueprints for the unimaginably vast array of self-assembling nanomachines that make our body work, which are called proteins. Once a protein is made, it does not propagate any information back to DNA; DNA alone encodes the genome. However, it is possible for RNA to modify DNA. This is how HIV installs itself as a rootkit in the cell, which will be covered in a future post. >The nucleus is the cell's library, storing the delicate strands of DNA and protecting them from the rigors of the cytoplasm. Much of the DNA is wrapped around histone proteins to form small nucleosomes (yellow four-legged proteins) that compact and protect the DNA. When the DNA is needed, it is unwrapped, unwound, and read by RNA polymerase (large orange protein engulfing the yellow strand) to create a messenger RNA (thin purple rope). The RNA molecules are then processed: capping enzymes protect one end and polyadenylate polymerase adds a repeated string of adenine nucleotides to the other end, which are then protected by the polyadenylate binding protein. Our RNA must also be edited by large splicosome complexes (large light purple protein) to remove intron regions that do not encode proteins. Once the RNA is properly edited, it is delivered out of the nucleus through nuclear pore complexes (large green structure). These pores span the double-layered nuclear membrane and control the traffic of a diverse collection of importin proteins (purple protein traversing the large green structure), which carry other molecules in and out. The nuclear membrane is strengthened inside by criss-crossed layers of lamin protein filaments (in blue) (1,000,000 X) Here is the zoomed-out context for this illustration; it shows the leftmost part of the rectangular colored box in this human blood plasma cell (10,000 X): 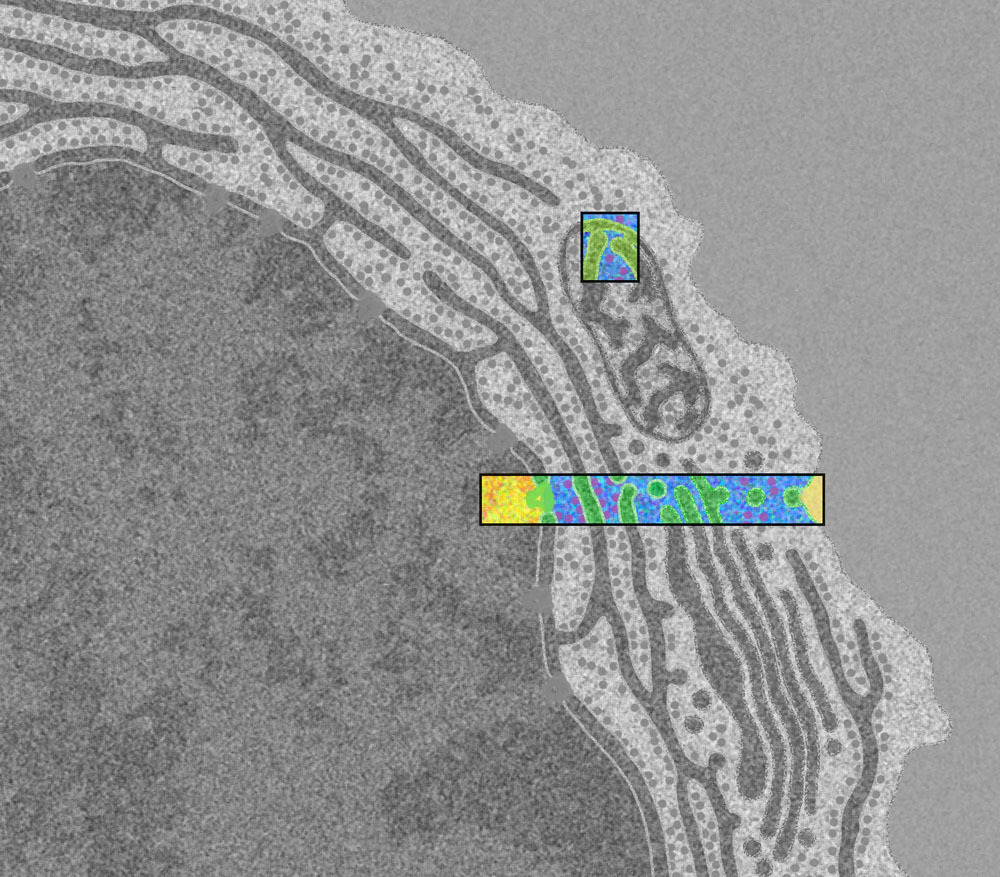 Future posts will move from left to right along this cross section, following the stages of the central dogma.

To make solar power viable, we need a solution for overnight energy storage. Batteries are complicated. Do you know what isn't? Water go up. 

Source: [*The Machinery of Life*](https://ccsb.scripps.edu/goodsell/machinery-of-life-reducedillustrations/) by David S. Goodsell >When we look closely at an *Escherichia coli* cell, we observe a bustle of organized activity. This illustration shows a portion of a cell magnified to where individual molecules may be seen. Only the large biomolecules are shown, including proteins, nucleic acids, polysacchandes, and lipid membranes. The spaces in between are filed with water, sugars, nucleotides, amino acids, metal ions and many other small molecules, as shown below (1,000,000 X) 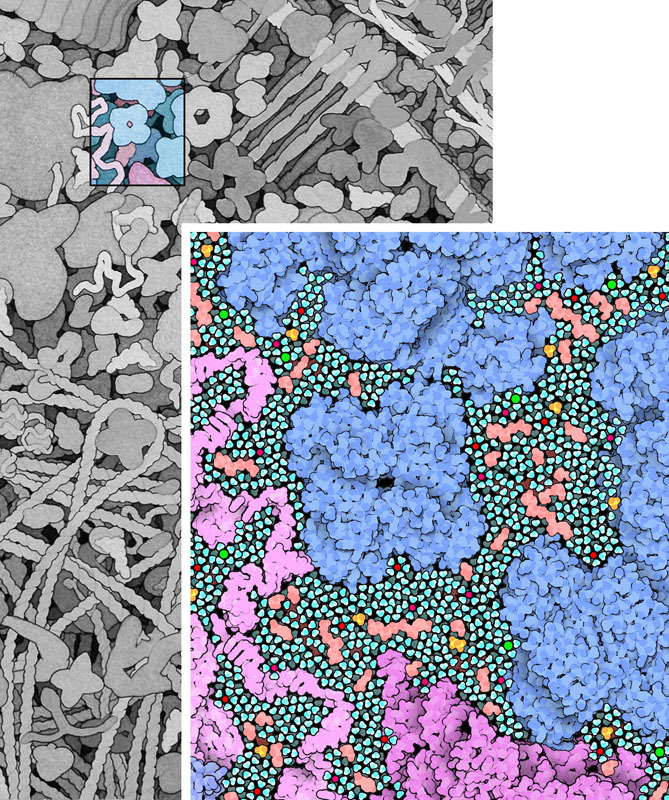 >**Water and Small Molecules** This illustration shows a small portion of the cell at a higher magnification, showing the crowding of small molecules between the larger proteins and nucleic acids. Amino acids, sugars, ATP, and many other small organic molecules are shown in pink. Metal ions are shown in bright red, phosphate ions are in yellow and orange, and chloride ions are in green. Al of the remaining space is filled with water molecules, colored turquoise (5,000,000 X) Here's the illustration in a larger context: 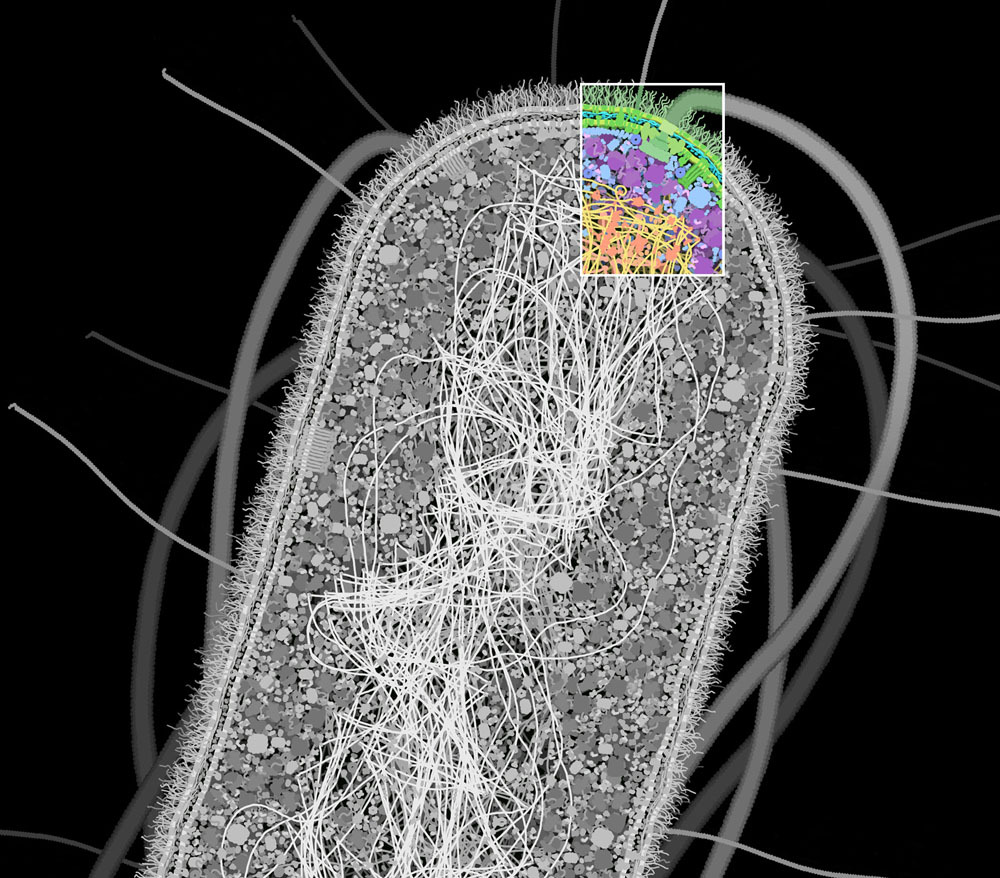 **Cellular Propellers** >The small size of *Escherichia coli* cells has a profound effect on the way that they interact with their surroundings. For them, gravity is not the overwhelming force that it is in our lives. Instead, the constant press of surrounding water is far more important. At small scales, water is not the flowing liquid that it is to us. The surface tension of water is a familiar example: small insects can skate along the surface of a pond, but if we try, the weight of our enormous bodies overwhelms the gentler forces of water and we plunge to the bottom. For cells, these differences are even greater. Cells live in a world of thick, viscous water, almost oblivious to gravity. When moving from place to place, most of their energy is spent trying to push through the gooey liquid, not in lifting their weight up from the ground. >For example, Howard Berg presented a surprising observation in his 1976 lecture "Life at Low Reynolds Number." *Escherichia coli* cells swim using long corkscrew-shaped flagella, which act like propellers. The cells push their way through the water, typically moving about 30 um/s (30 um is 10 or 15 times the length of the cell). But, when they stop turning the flagella, they don't keep coasting along the way a ship or submarine would. Instead, the surrounding water instantly stops them in less than the diameter of a water molecule. >The flagellar motor is one of the wonders of the biomolecular world. The motor spans the entire cell wall, rotating at speeds of up to 18,000 revolutions per minute. Each rotation is powered by the flow of about 1000 hydrogen ions across the inner membrane. Amazingly, the motor can turn the flagellum in either direction, clockwise or counterclockwise, on demand. When it turns in one direction, all of the flagella on the cell get tangled into a bundle, and together they propel the cell through the surrounding water. If the motor switches direction, however, the flagella separate and flail in different directions, causing the cell to stop and tumble in place. >Bacteria are faced with a serious problem with navigation. Since they are so tiny, they have no way to aim themselves in the right direction. A bacterial cell can't look at a distance and see what direction food lies. Instead, *Escherichia coli* cells use an effective combination of the swimming and tumbling properties of their flagella. Each cell uses an array of sensors to determine the level of food in the immediate vicinity. Then it swims in a random direction, and measures the level of nutrients there. If the levels are increasing, it keeps swimming in that direction, since things are getting better. If not, the sensory array sends a signal to the flagellar motor, telling it to reverse direction. This causes the cell to tumble, picking a new (and hopefully better) direction to swim. In the rich, watery environment of our intestines, this almost random approach is adequate to keep the cell nourished.

Taken from the book [*The Machinery of Life*](https://ccsb.scripps.edu/goodsell/machinery-of-life-reducedillustrations/) by David S. Goodsell As we all know, the mitochondria is the powerhouse of the cell. >Unlike simpler bacterial cells, our cells are filled with compartments that perform different duties. ATP synthesis is the primary task performed by the mitochondria, shown here in cross-section. The enzymes of the citric acid cycle (purple & blue) are found in the innermost space and the folded inner membrane provides the necessary separation of spaces needed to create the electrochemical gradient that powers [ATP synthase](https://hexbear.net/post/3554975) (green mushroom shape). This membrane is one of the most protein-concentrated membranes found in our cells: by some estimates, three quarters of the membrane is composed of protein, with just enough lipid to hold it together. Compare this illustration with the illustration of *Escherichia coli* in [this previous post](https://hexbear.net/post/3561041), and notice that the mitochondrion includes very similar machinery for protein synthesis, complete with DNA, RNA, and ribosomes. It does not make all of its own proteins, however, and some must be transported from the cytoplasm by specialized transport proteins (light green crown shape) in the inner and outer membranes (1,000,000 X) More on the advanced compartmentalization of human cells and the odd origin of mitochondria: >The human body is composed of trillions of cells. Just like a bacterial cell, each one of our cells (with a few exceptions) has DNA, polymerases, and ribosomes for building proteins based on genomic information. Each cell is filled with a collection of enzymes for making and breaking the many molecules needed for growth and energy production. Each cell is surrounded by a sturdy cell membrane filled with channels, pumps, and sensors. Each of our cells, however, is much larger and far more complex than a bacterial cell. Where bacteria are built to be fast and efficient, our cells are made to perform complex and specific tasks, and they are made to last and perform these tasks for years. >By looking at the similarities and differences between bacterial cells and animal cells, scientists have discovered that a breakthrough occurred in the evolution of life about 1.5 billion years ago. Simple, bacteria-like cells had probably been around for at least 2 billion years, and most of the basic molecular machinery of life had been perfected. Then, a new design of the cell body appeared: larger cells filled with many small, separate compartments, each surrounded by its own watertight membrane. The proliferation of these new cells signaled a major break in the evolution of cells, and in the years since then, two distinct family lines have evolved. The simple cells, with their molecular machinery jumbled together in one compartment, are the distant ancestors of the bacteria and archaebacteria. The new compartmented cells gave rise to all other forms of life, including protozoa, fungi, plants, and animals. >Surprisingly, when we look under the microscope, some of these compartments look similar to whole bacterial cells. The mitochondria in each of our cells have a similar shape, size, and composition as an *Escherichia coli* cell. For instance, mitochondria are surrounded by two membranes. The outer membrane is studded with proteins reminiscent of bacterial porins. The inner membrane, pleated and folded in mitochondria, is filled with proteins of transport and energy production similar to the bacterial proteins. Even more surprising, when you look inside a mitochondrion, it has its own DNA, transfer RNA, and ribosomes, all busily constructing mitochondrial proteins. These remarkable similarities have prompted a theory of the origin of mitochondria (and chloroplasts) that is now widely accepted. It is thought that a bacterial cell took to living inside another cell in the distant past, perhaps entering as a parasite or perhaps surviving the process of being eaten. The bacterium continued to live in this comfortable, sheltered environment, living and reproducing inside the cell. Gradually, both the engulfed cell and the cellular host became increasingly reliant on each other, dividing up the tasks of living. Today, our mitochondria continue to divide and reproduce inside our cells, but they rely on the rest of the cell for many of their essential proteins and nutrients. Here is a zoomed-out view (10,000 X) of a blood plasma cell under a microscope; the topmost box shows this post's headline illustration in context. The sheer scale of each ordinary human cell is incredible! And we have *trillions* of them! 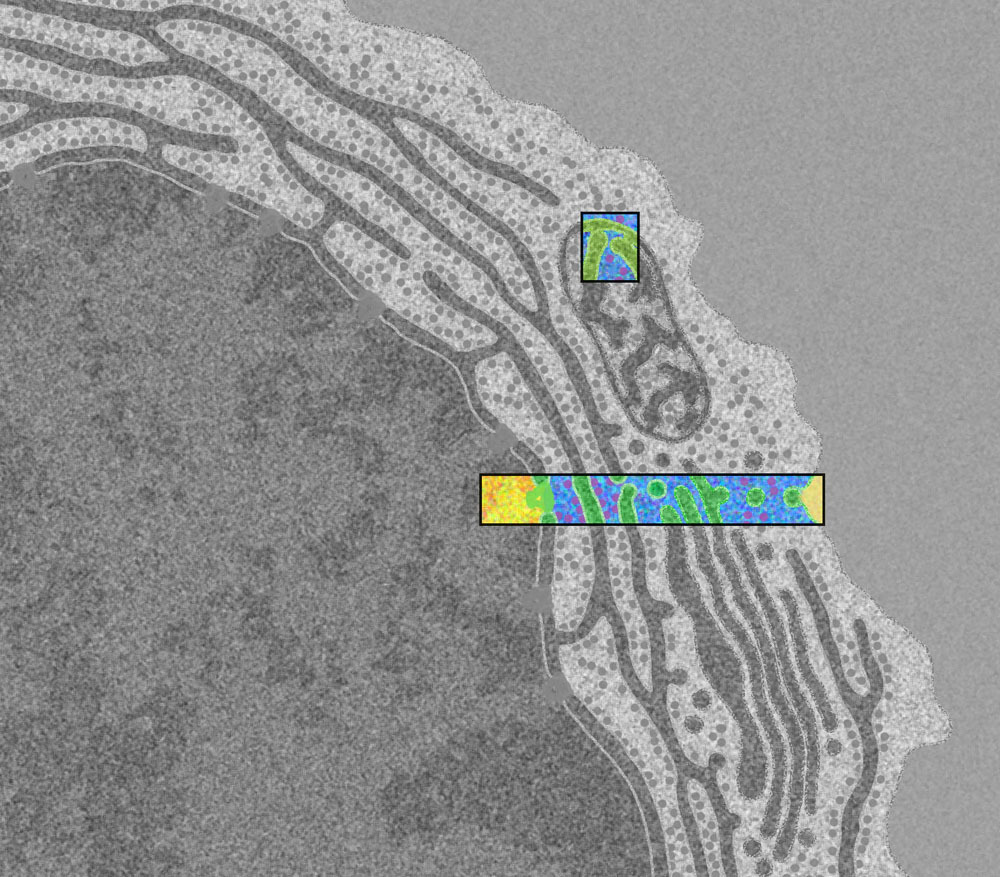

From [*The Machinery of Life*](https://ccsb.scripps.edu/goodsell/machinery-of-life-reducedillustrations/) by David S. Goodsell. >ATP synthase is a molecule-sized generator that converts electrochemical energy into chemical energy. It is composed of two rotary motors connected together by an asymmetric axle. Flow of hydrogen ions through the bottom motor, which is embedded in a membrane, turns the large cylindrical rotor. This is connected to the axle, forcing it to turn inside the second motor at the top. With each turn, the axle distorts the subunits in the upper motor, catalyzing a reaction that makes the unstable phosphate-phosphate bond in ATP (5,000,000 X) ATP Synthase is incredibly cool because it is probably the closest thing our body has to an honest-to-god recognizable nanomachine as humans would conceive of it. It can pump & rotate in both directions! If there is an electrochemical gradient across the membrane it sits on, it can sap the potential energy of that gradient to synthesize ATP to store energy for later as it lets ions across the membrane. Alternatively, it can consume ATP to pump ions in the opposite direction, creating an electrochemical gradient itself! There are a lot of cool animations you can find online, like this one: https://www.youtube.com/watch?v=kXpzp4RDGJI How are electrochemical gradients created in the first place? Here's how plants do it with photosynthesis: 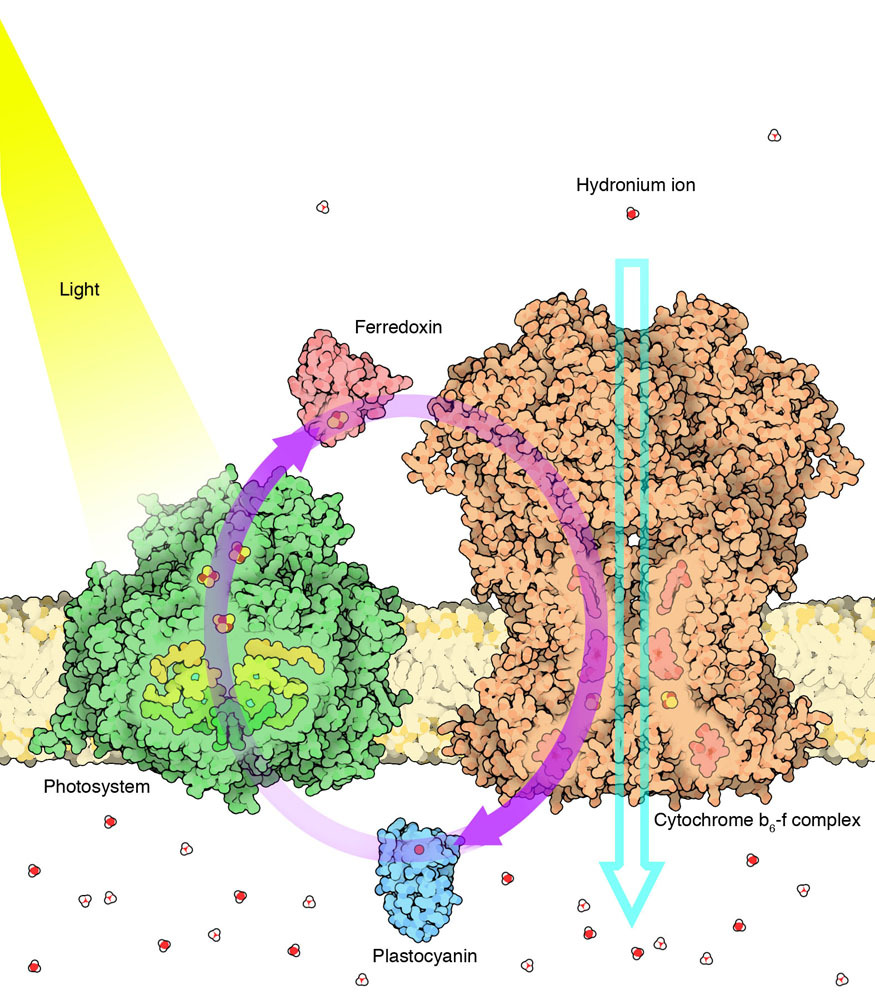 >**Cyclic Photophosphorylation** Plants use the energy of light to make ATP, but they must go through many energy transformations to do it. The process starts with a photosystem protein that captures individual photons of light, and uses the energy of these photons to create high-energy electrons. These electrons then flow through several proteins, hopping through a chain of iron ions and copper ions and slowly losing their energy until they return to the photosystem, ready for another charge from light. As the electrons flow through the cytochrome b₆-f complex, they are used to power the pumping of hydrogen ions across the membrane, creating an electrochemical gradient. This gradient is finally used to power ATP synthase (5,000,000 X) As always, remember that none of the things we are talking about have any willpower or desire - it's all just physics and random interactions and energy being moved around. Also remember that none of these systems were designed, but instead came into being through natural selection.

>A spider sputter-coated in gold to prepare it as a specimen for scanning electron microscopy. > >A conductive coating is needed to prevent charging of a specimen with an electron beam in conventional SEM mode (high vacuum, high voltage). > >This item was on display at the Australian Museum in Sydney, New South Wales, Australia. —Wikipedia

Illustration from [*The Machinery of Life*](https://ccsb.scripps.edu/goodsell/machinery-of-life-reducedillustrations/) by David S. Goodsell >Molecules constantly diffuse within the interior of cells, randomly bumping from place to place. This illustration shows several snapshots from a computer simulation of a protein and a sugar molecule diffusing inside a bacterial cell. The path of the protein is shown in blue and the path of sugar is shown in red. They start at opposite ends and explore much of the space inside before they reach each other. I really like this because it illustrates, more than anything else, that our biomolecules don't "want" to do anything, go some place, bind somewhere; it's just physics and unbelievable quantities of random interactions. Excerpt from the text: >One basic thing remains the same at our size and at molecular size: the solidity of matter. At the scale of molecules, we do not need to worry too much about the odd things that happen with quantum mechanics: to a first approximation, molecules have a definite size and shape, and it is perfectly fine to imagine them bumping into each other and fitting together if the shapes match. If we look closely, their edges may be a bit fuzzy, but for most purposes, we can think of them as physical objects like tables and chairs. >Other properties, however, are very different when we enter the molecular world. For instance, molecules are so small that gravity is completely negligible. The motions and the interactions of biological molecules are completely dominated by the surrounding water molecules. At room temperature, a medium-sized protein travels at a rate of about 5 m/s (the speed of a fast runner). If placed alone in space, this protein would travel its own length in about a nanosecond (a billionth of a second). Inside the cell, however, this protein is battered from all sides by water molecules. It bounces back and forth, always at great speed, but takes a long time to get anywhere. When surrounded by water, this typical protein now requires almost a thousand times longer to move a protein-sized distance. >Imagine a similar situation in our world. You enter an airline terminal and want to reach a ticket window on the far side of the room. The distance is several meters—a distance comparable to your own size. If the room is empty, you dash across in a matter of seconds. But imagine instead that the room is crowded full of other people trying to get to their respective windows. With all the pushing and shoving, it now takes you 15 minutes to cross the room! In this time, you may be pushed all over the room, perhaps even back to your starting point a few times. This is similar (although molecules do not have a goal in mind) to the contorted path molecules take in the cell. >You might ask how anything ever gets done in this chaotic world. It is true that the motion is random, but it is also true that the motion is very fast compared to the motion in our familiar world. Random, diffusive motion is fast enough to perform most of the tasks in the cell. Each molecule simply bumps around until it finds the right place. >To get an idea of how fast this motion is, imagine a typical bacterial cell and place an enzyme at one end and a sugar molecule at the other. They will bump around and wander through the whole cell, encountering many molecules along the way. On average, though, it will only take about a second for those two molecules to bump into each other at least once. This is truly remarkable: this means that any molecule in a typical bacterial cell, during its chaotic journey through the cell, will encounter almost every other molecule in a matter of seconds. So as you are looking at the illustrations in this book, remember that static images give only a single snapshot of this teeming molecular world.
The beginning of the article > Who knew a dragon’s tongue could be so long? > > Astronomers announced last week that they had discovered a black hole spitting energy across 23 million light-years of intergalactic space. Two jets, shooting in opposite directions, compose the biggest lightning bolt ever seen in the sky — about 140 times as long as our own Milky Way galaxy is wide, and more than 10 times the distance from Earth to Andromeda, the nearest large spiral galaxy. > > Follow-up observations with optical telescopes traced the eruption to a galaxy 7.5 billion light-years away that existed when the universe was less than half its current age of 14 billion years. At the heart of that galaxy was a black hole spewing energy equivalent to the output of more than a trillion stars. > > “The Milky Way would be a little dot in these two giant eruptions,” said Martijn Oei, a postdoctoral researcher at the California Institute of Technology. Dr. Oei led the team that made the discovery, which was reported in Nature on Sept. 18 and announced on the journal’s cover with an illustration reminiscent of a “Star Wars” poster. The astronomers have named the black hole Porphyrion, after a giant in Greek mythology — a son of Gaia — who fought the gods and lost. > > The discovery raises new questions of how such black holes could affect the evolution and structure of the universe. Wikipedia: [Porphyrion (radio galaxy)](https://en.wikipedia.org/wiki/Porphyrion_(radio_galaxy))

**The Immune System Piercing a Bacterial Cell Wall** Our blood contains proteins that recognize and destroy invading cells and viruses. This illustration shows a cross section through a bacterial cell wall (lower half, in greens, blues and purples) being attacked by proteins in the blood serum (at the top, in yellows and oranges). Y-shaped antibodies begin the process by binding to the surface of the cell, and are in turn recognized by the six-armed protein at upper center. This begins a cascade of actions that ultimately lead to the formation of a membrane attack complex, shown here piercing the cell wall of the bacterium (1,000,000 X). [Source](https://ccsb.scripps.edu/goodsell/machinery-of-life-reducedillustrations/)
thanks for showing me this [@ElChapoDeChapo@hexbear.net](https://hexbear.net/u/ElChapoDeChapo)
So I recently found that the University of Minnesota has an Open Textbook Library of textbooks that are licensed to be freely downloaded and distributed and figured I'd post the link here for anyone else who likes studying from textbooks and teaching themselves as I do (I've reread my own textbooks repeatedly from years ago because I'm apparently a bit odd as I've been repeatedly told). Is this the right community for that or are there any others that would be good to cross-post to? Was recently reading the sociology textbooks because those are the ones that seem to reference Marx and Engels a bunch.
 theconversation.com
theconversation.com
You mean it's all just pension fraud?  >In Okinawa, the best predictor of where the centenarians are is where the halls of records were bombed by the Americans during the war. 
 www.popsci.com
www.popsci.com
Is it possible to learn this power? 
In places where bat populations crashed, farmers sprayed more insecticides, and baby mortality spiked - 05 SEP 2024 - Erik Stokstad https://www.science.org/content/article/my-jaw-dropped-bat-loss-linked-death-human-infants In 2006, bats throughout New England began dying en masse from a mysterious and incurable fungal disease called white nose syndrome. Over the next decade, their populations plummeted—and humans living nearby suffered, according to a new study. With fewer predators around, insect numbers increased, leading to farmers spraying about 31% more pesticides, researchers report this week in Science. At the same time, infant mortality in counties increased by 8%. The authors link those deaths to the rise in the use of insecticides, which are known to be dangerous, especially for fetuses and infants. That link is a “pretty dramatic claim that’s going to get a lot of attention,” says Paul Ferraro, a sustainability scientist at Johns Hopkins University who was not involved with the new work. The study, he says, is the “most convincing evidence to date” linking economic and health impacts with dramatic losses of a wild species. Bats are good to have around a farm. They provide free pest control, with some species consuming 40% of their body weight each night in insects. The value of this service has been estimated at between $4 billion and $53 billion per year. So, it’s logical to assume farmers might compensate for a loss of bats by spraying more insecticides, says Winifred Frick, chief scientist at Bat Conservation International. Making a watertight case for that assumption, however, isn’t easy. Eyal Frank, an economist at the University of Chicago, realized that the decline of bat populations due to white nose syndrome presented a kind of natural experiment. Because the disease appeared suddenly and spread rapidly, Frank could compare outcomes in counties where bat populations plummeted with those in similar counties that had not yet been struck. In the first year after an area was hit by the disease, farmers tended to spray an extra kilogram of insecticide per square kilometer, Frank found. After 5 years, they were spraying 2 kilograms more than before—a 31% increase on average. At the same time, fungicide and herbicide rates did not increase, suggesting the need for more intensive insect control drove the insecticide change. Frank also looked at infant mortality in all the counties. In places where the bat populations had crashed, deaths due to accident or homicides stayed the same. But other deaths, such as those caused by disease or birth defects, rose 8%. In counties with healthy bat populations, the numbers didn’t shift one way or another. “My jaw dropped,” Frick says. Several lines of evidence connect pesticides and other agrochemicals to human health risks. Although government regulators assess the potential dangers of these compounds before approving them—and set safety guidelines for their use—farm workers and bystanders can still get exposed when these compounds drift away from a farm or end up in groundwater. Epidemiological studies have linked certain compounds to developmental problems in infants and children, for example. Insecticides, which are often neurotoxic, are often of particular concern. The increase in deaths is “huge,” says Tracey Woodruff, an environmental health scientist at the University of California San Francisco. The connection is plausible and concerning, she says. In an earlier study, she found an increase in infant mortality of similar magnitude due to worsening air pollution. But a puzzling fact about the new study is that other aspects of infant health, such as birth weight, did not correlate with the bat declines. Still, other confounding factors might have contributed to the rise in mortality, Ferraro notes. “I wouldn’t change public policy based on this one study.” Frick says there are signs that some populations of bats are beginning to recover, but it could take decades to return to their previous abundance. Her organization is trying to help by setting up lights to attract more insects to winter hibernation sites to make sure bats are eating their fill. Other conservationists are experimenting with changing ventilation of abandoned mines to make their temperature more favorable to roosting bats. Meanwhile, the fungus that causes white nose Syndrome continues to spread into the western United States, including California, a major agricultural region. doi: 10.1126/science.zu56w28

https://www.livescience.com/animals/birds/mice-on-remote-island-that-eat-albatrosses-alive-sentenced-to-death-by-bombing-scientists-decree >Invasive mice are devouring albatrosses alive on a remote island in the Indian Ocean, so conservationists have come up with an explosive solution — "bombing" the mice. > >Mice have been wreaking havoc on Marion Island, between South Africa and Antarctica, for decades. Humans accidentally introduced the mice in the 19th century, and the rodents have since developed a taste for wandering albatrosses (Diomedea exulans) and other threatened seabirds. > >The Mouse-Free Marion Project, a collaboration between the South African government and BirdLife South Africa, is trying to raise $29 million to drop 660 tons (600 metric tons) of rodenticide-laced pellets onto the island in winter 2027, AFP news agency reported on Saturday (Aug. 24). > >The project plans to send a squad of helicopters to drop the pellets. By striking in winter when the mice are most hungry, the conservationists hope to eradicate the entire mouse population of up to 1 million individuals.
> An interdisciplinary team of researchers put a culture of the edible mushroom species *Pleurotus eryngii* (also known as the king oyster mushroom) in control of a pair of vehicles, which can twitch and roll across a flat surface. >By applying algorithms based on the extracellular electrophysiology of *P. eryngii* mycelia and feeding the output into a microcontroller unit, the researchers used spikes of activity triggered by a stimulus – in this case, UV light – to toggle mechanical responses in two different kinds of mobile device. https://youtu.be/5ZkkaM54RH8 https://www.science.org/doi/10.1126/scirobotics.adk8019
 inv.nadeko.net
inv.nadeko.net
Interesting video, this is far from resolving all the world's issues of course, this is essentially throwing a bunch of plastic in a tube and microwaving it to extreme temperatures, but it can create a ton of graphene (30mg plastic -> 5mg graphene conversion). The proposed use for this graphene is in cement mixes in order to toughen concrete and reduce wear on roads and structures. > The technique uses flash Joule heating (FJH) to heat carbonaceous materials to temperatures over 3000 K in ∼100 ms, producing >90% yields of high quality turbostratic FG (tFG). The high temperatures of FJH result in high purity tFG, since much of the non-carbon atoms are removed through sublimation Using this much energy is of course problematic, sure, it can get rid of the plastic, but we are largely burning fossil fuels to create this energy. It takes 400-600w of electricity to produce this much graphene, which is certainly a breakthrough for graphene production, at least, because of how labor cheap this is. Its also much cheaper electricity wise than pretty much every pre-existing recycling method, which is certainly a good thing. > In this study, the goal is to broaden the application of APMP to solid precursors, specifically converting microplastics into graphene. In contrast to the traditional method of initiating graphene production from gaseous-phase products, this approach involves the transformation of PE microplastics into gases such as methane, ethylene, and ethane, and then converting them into graphene within the plasma, all in one step. Furthermore, the advantages of microwave-based technologies in terms of energy consumption and cost compared to conventional techniques for recycling or upcycling polymers can be found in recently reported studies.[27-29] Following the successful synthesis of graphene, we also showcase its effectiveness in adsorbing perfluorooctanoic acid (PFOA), facilitated by ultrasonication. The beauty of this is you don't have deep concerns about offgassing, the plasma captures the most harmful vapors and flash converts them to a graphene powder, which resolves a concern of harmful contaminates to local areas of production. It will mostly offgas H2, Carbon monoxide, and Co2, though the paper doesnt seem to discuss the quantities of each gas it produces. Co2 offgassing is obviously problematic itself if that quantity is very high, you're essentially turning a lot of the 'stored' co2 in plastic into gas in the atmosphere. Based on similar studies, it does seem that it will off gas far more H2 than CO or CO2 though --- tl:dr: some scientists got a tube filled it with argon and smashed up microplastics, chucked it in a 500 watt microwave, and got a way to make a lot of money off of graphene synthesis
There aren't many comments but they are very good. https://www.nytimes.com/2024/08/30/science/iceland-volcano-eruption-lava.html#commentsContainer
The two astronauts will remain on the ISS until February 2025, when they'll return with two astronauts on the SpaceX Crew-9 mission that's arriving at the ISS next month.
It's a NYT article. The actual subheader was a too long. > A study adds strong evidence to the hypothesis that the deadly rock came from a family of objects that originally formed well beyond the orbit of the planet Jupiter. \--- > A team led by Mario Fischer-Gödde, a research scientist at the University of Cologne in Germany, has bolstered that case with the help of the rare element ruthenium. Ruthenium is abundant in asteroids but extremely scarce in Earth’s crust, making it a handy bellwether of past impacts by space rocks. The team searched for isotopes of ruthenium in the geological remnants of the Chicxulub impact.
 www.theverge.com
www.theverge.com
Most of the data we have looks at the health effects of radiation like gamma rays and X-rays, which cause damage across the body in a “uniform, spray-bottle kind of pattern,” explained radiation biologist Greg Nelson, who advises NASA on radiation health research. But galactic cosmic rays move through the body in a straight line, like a track. “So you concentrate damage on a microscopic scale, and that damage, because it’s so concentrated, is much more difficult for the body to repair,” Nelson said. This type of space radiation isn’t like the low-dose exposure of a chest X-ray. Instead, imagine a charged particle traveling at nearly the speed of light, firing straight through your brain, perturbing 10,000 cells all in a row, all within a microsecond. It’s not necessarily damaging those cells, but it is activating them in a highly unusual way. And we don’t yet know what that does. “It’s that feature, that we would call track structure, that lends itself to the possibility of new and different effects occurring,” Nelson said. While most radiation on Earth can cause cancer by breaking apart DNA, the latest research suggests these charged particles could be damaging the brain in an entirely different way, such as by disrupting the connections between neurons or the mitochondria within

https://link.springer.com/article/10.1007/s44187-024-00136-1
science
!science@hexbear.netWelcome to Hexbear's science community!
Subscribe to see posts about research and scientific coverage of current events
No distasteful shitposting, pseudoscience, or COVID-19 misinformation.










